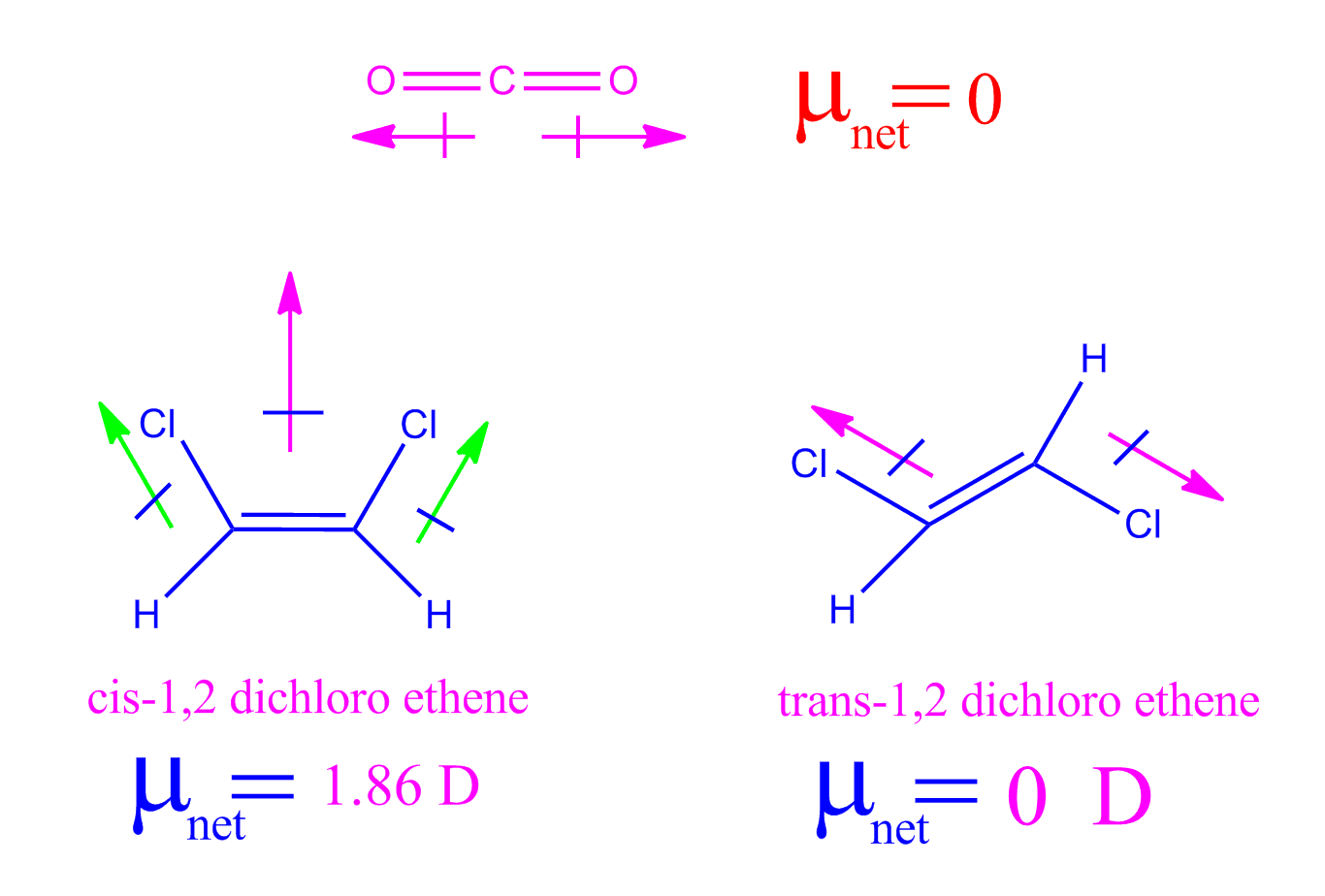
What Is A Net Dipole
1.8. intermolecular forces Must polar bonds give rise to polar molecules? and, why is water a Dipole moment electric cp magnetic physics violation field direction spin neutron edm quantum formula griffin mdm oment scienceabc
PPT - Lecture 21: Ionic to Covalent PowerPoint Presentation, free
Dipole forces chemistry covalent molecules intermolecular interactions bonds attractive compounds organic liquids shape polar molecular repulsive attraction atoms figure occur Determining if a molecule has a net dipole and intro to ionic bonding Dipole covalent forces intermolecular interactions chemistry molecules hydrogen dipoles bonds bonding structure oxygen libretexts textbook chem mol
Dipole moments
What is a dipole? what is dipole moment?Dipole moment molecule hcl direction chemistry bond formula moments definition magnitude vector explanation acid has hydrochloric arises provided below Molecular polarity molecular structureBond dipole moment dipoles pngfind.
Dipole water moment chemistry moments polarity molecular molecules physics bond positive negative arrow electric dipoles molecule chemical structure file vectorDipole moment definition, formulas & examples Dipole moment bond moment group moment and influence of dipole momentMolecular dipole.

Dipole moment moments bond ionic molecule covalent has molecular bonding if which lecture ppt presentation determining chemical polar concepts chapter
Dipole moment examples definition example equation molecule moments ammonia polar lesson study nh3 video negativeDipole moment and its applications – toppr bytes Dipole molecule ifDipole moment.
Solved which of the following molecules have a net dipoleWhy polar molecule water bonds dipole h2o molecules moment partial positive permanent must hydrogen rise give charges not atoms Dipole explanationDipole moment bond zero group influence value tetra benzene carbon why.

Dipole molecules moments polar bonds molecular geometry chemistry structure bond polarity vsepr covalent organic bonding moment does each model libretexts
In the case of water, the two bond dipoles arrows areDipole molecular polarity molecule chemistrysteps affects Net dipole momentDipole moment polar nonpolar dipoles bond polarity molecular zero overall molecule nonzero structure h2o chemistry has do but not magnitude.
11.3: dipole-dipole forcesDipole molecules 7.the dipole-moment of system is?? how to find..explanation please.